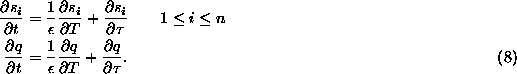
 ,
while the rest of the concentrations,
,
while the rest of the concentrations, We also expand the s and q concentrations as:
Inserting Eqs. (8) and (9)
in Eqs. (1)-(2)
and equating terms with equal powers of ![]() we find:
we find:
To ![]() :
:
To ![]() :
:
Using relation (7) we can rewrite Eqs. (10)-(13) as:
for ![]() .
.
As mentioned before, in principle, ![]() (
( ![]() ) and q depend on both T and
) and q depend on both T and ![]() . However, due to the separation of timescales, we can first integrate
the equations in T treating
. However, due to the separation of timescales, we can first integrate
the equations in T treating ![]() as an independent variable that is held constant
as an independent variable that is held constant  .
We do that with Eqs. (14)-(15),
which then can be treated as a set of n+1 ordinary differential
equations. The solutions will be of the form:
.
We do that with Eqs. (14)-(15),
which then can be treated as a set of n+1 ordinary differential
equations. The solutions will be of the form:
where ![]() corresponds to a fixed point solution of the set (14)-(15),
i.e., they satisfy:
corresponds to a fixed point solution of the set (14)-(15),
i.e., they satisfy:
with ![]() (
( ![]() ) and
) and ![]() independent of T. From (20) we expect
to find algebraic relations of the form
independent of T. From (20) we expect
to find algebraic relations of the form
that define n-dimensional sets of fixed points in the (n+1)-dimensional
space of concentrations, ![]() . Here the main difference between the cases of reversible and irreversible
reactions arises. In the case of one reaction of the form (4)
with
. Here the main difference between the cases of reversible and irreversible
reactions arises. In the case of one reaction of the form (4)
with ![]() we obtain only one relation given by:
we obtain only one relation given by:
On the other hand, if k'=0, then (20)
implies that at least one concentration, ![]() , is equal to zero. We schematically depict both situations in Figs. 1
(a) and (b), where we have plotted the surfaces determined by condition
(20) in the case of three species,
, is equal to zero. We schematically depict both situations in Figs. 1
(a) and (b), where we have plotted the surfaces determined by condition
(20) in the case of three species, ![]() ,
, ![]() and Q.
and Q.
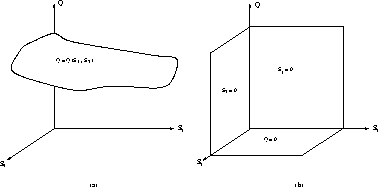
Figure: (a) Manifold defined by the algebraic relation (22)
in the case of one fast reversible reaction involving three species, ![]() ,
, ![]() and
and ![]() . (b) Similar to (a) but for one irreversible reaction involving
. (b) Similar to (a) but for one irreversible reaction involving ![]() ,
, ![]() and Q. In this case three surfaces of fixed points for the fast
dynamics exist:
and Q. In this case three surfaces of fixed points for the fast
dynamics exist: ![]() ,
, ![]() and Q=0.
and Q=0.
We discuss the ![]() case first. Since there are no feeding terms in Eqs. (14)-(15),
we expect the dynamical system (14)-(15)
to be dissipative. Therefore, we expect the existence of attractors. On
the other hand, as shown in Sec. III, due to the existence of several constants
of motion, the only possible attractors are fixed points. In particular,
we assume that all the fixed points defined by (21)
are stable
case first. Since there are no feeding terms in Eqs. (14)-(15),
we expect the dynamical system (14)-(15)
to be dissipative. Therefore, we expect the existence of attractors. On
the other hand, as shown in Sec. III, due to the existence of several constants
of motion, the only possible attractors are fixed points. In particular,
we assume that all the fixed points defined by (21)
are stable  .
Then, for almost every initial condition, the functions
.
Then, for almost every initial condition, the functions ![]() and
and ![]() will become negligible in front of
will become negligible in front of ![]() and
and ![]() , respectively, as T increases. Which fixed point on the N-dimensional
space defined by (20) is initially approached
depends on the initial condition (see Sec. III). Given one initial condition,
the system approaches very fast (on the fast timescale) this initial ``fixed
point'', which is actually a fixed point for the fast timescale dynamics.
Then, the slow dynamics moves the system on the N-dimensional space
defined by (20). In other words, on the fast
timescale, the concentrations
, respectively, as T increases. Which fixed point on the N-dimensional
space defined by (20) is initially approached
depends on the initial condition (see Sec. III). Given one initial condition,
the system approaches very fast (on the fast timescale) this initial ``fixed
point'', which is actually a fixed point for the fast timescale dynamics.
Then, the slow dynamics moves the system on the N-dimensional space
defined by (20). In other words, on the fast
timescale, the concentrations ![]() and q approach the slowly varying functions
and q approach the slowly varying functions ![]() and
and ![]() . These are the slowly varying portions of the concentrations that we are
interested in. The algebraic relation (21)
implies that
. These are the slowly varying portions of the concentrations that we are
interested in. The algebraic relation (21)
implies that ![]() depends on
depends on ![]() (and
(and ![]() ) through the
) through the ![]() (and
(and ![]() ) dependence of the concentrations
) dependence of the concentrations ![]() ,...,
,..., ![]() . It also means that not all of the slowly varying concentrations are independent,
and this allows the reduction of the number of relevant dynamic equations.
. It also means that not all of the slowly varying concentrations are independent,
and this allows the reduction of the number of relevant dynamic equations.
We now turn to Eqs. (16)-(17).
Replacing the solutions (18)-(19)
and neglecting the terms ![]() and
and ![]() in front of
in front of ![]() and
and ![]() respectively, we find:
respectively, we find:
where the partial derivatives ![]() ,
, ![]() ,
, ![]() and
and ![]() must be evaluated at the fixed point
must be evaluated at the fixed point ![]() , and
, and ![]() should be written as a function of
should be written as a function of ![]() using relation (21) ((22)
in our case).
using relation (21) ((22)
in our case).
So far we have not talked about the evolution equations for the v
concentrations. Inserting the solutions (18)-(19)
in (3) and neglecting the terms ![]() and
and ![]() in front of
in front of ![]() and
and ![]() as before, the form of Eqs. (3) remains
the same, with the only difference that
as before, the form of Eqs. (3) remains
the same, with the only difference that ![]() and q are to be replaced by
and q are to be replaced by ![]() and
and ![]() . This is consistent with our previous assumption that the v concentrations
only vary on the slow timescale,
. This is consistent with our previous assumption that the v concentrations
only vary on the slow timescale, ![]() . In this way, all the terms on the right-hand-side of Eqs. (23)-(24)
only depend on
. In this way, all the terms on the right-hand-side of Eqs. (23)-(24)
only depend on ![]() , while the ones on the left-hand-side depend both on
, while the ones on the left-hand-side depend both on ![]() and T. Thus, these equations can be separated in two parts:
and T. Thus, these equations can be separated in two parts:
and
with ![]() and
and ![]() unknown functions of
unknown functions of ![]() .
.
In order to get rid of these unknown functions, we multiply each of
Eqs. (23) by ![]() and subtract Eq. (24). We get n
equations of the form:
and subtract Eq. (24). We get n
equations of the form:
As before, these equations can be separated in two:
with ![]() . These functions of
. These functions of ![]() must be treated as constants when solving Eqs. (25)-(26).
From Eq. (30) we see that if
must be treated as constants when solving Eqs. (25)-(26).
From Eq. (30) we see that if ![]() is different from zero, then
is different from zero, then ![]() will grow linearly in time with T. In this way this difference will
become too large, breaking up the asymptotic expansion (9).
Therefore, the only way to keep this expansion well-behaved it is to set
will grow linearly in time with T. In this way this difference will
become too large, breaking up the asymptotic expansion (9).
Therefore, the only way to keep this expansion well-behaved it is to set ![]() for al
for al ![]()
 .
We have the freedom to do it. In this way we guarantee that the solutions
of Eqs. (25)-(26),
on the fast timescale, approach slowly varying functions of time that remain
bounded for all times so that
.
We have the freedom to do it. In this way we guarantee that the solutions
of Eqs. (25)-(26),
on the fast timescale, approach slowly varying functions of time that remain
bounded for all times so that ![]() and
and ![]() . This let us conclude that
. This let us conclude that
Setting ![]() also leaves us with a closed set of n+N differential equations
for the slowly varying concentrations
also leaves us with a closed set of n+N differential equations
for the slowly varying concentrations ![]() ,
, ![]() and
and ![]() ,
, ![]() . Namely, we obtain the n equations for
. Namely, we obtain the n equations for ![]() inserting the algebraic relation (21)
in (31). They read:
inserting the algebraic relation (21)
in (31). They read:
for ![]() , with
, with ![]() given by (22). In particular, from (22)
we obtain that:
given by (22). In particular, from (22)
we obtain that:
Inserting (35) in (34) we obtain the equations:
which must be solved coupled to Eqs. (3)
with ![]() . As shown in Sec. III, given initial conditions for Eqs. (1)-(3)
we can determine unique values for
. As shown in Sec. III, given initial conditions for Eqs. (1)-(3)
we can determine unique values for ![]() (
( ![]() ) and
) and ![]() that satisfy the relation (21). These,
together with
that satisfy the relation (21). These,
together with ![]() , are the initial conditions for the reduced set of equations.
, are the initial conditions for the reduced set of equations.
We now repeat the calculation in the case of one fast irreversible reaction,
i.e., when ![]() and
and ![]() are of the form (5)-(6)
with k'=0. We introduce the two time variables, T and
are of the form (5)-(6)
with k'=0. We introduce the two time variables, T and ![]() , and the expansion (9) getting Eqs.
(10)-(13)
as before. But now, when we look for fixed point solutions of the ``fast''
system (10)-(11)
we observe that there are n+1 n-dimensional sets of fixed
points defined by
, and the expansion (9) getting Eqs.
(10)-(13)
as before. But now, when we look for fixed point solutions of the ``fast''
system (10)-(11)
we observe that there are n+1 n-dimensional sets of fixed
points defined by ![]() ,
, ![]() ,...,
,..., ![]() or q=0. As explained in Sec. III, if the dynamics is dissipative,
then there are always stable fixed points in some of those sets. As in
the
or q=0. As explained in Sec. III, if the dynamics is dissipative,
then there are always stable fixed points in some of those sets. As in
the ![]() case, given an initial condition for the system (10)-(11),
there is a unique stable fixed point that the system approaches eventually
in time. Without loss of generality let us call Q the species for
which the concentration is zero at this attracting fixed point (clearly
case, given an initial condition for the system (10)-(11),
there is a unique stable fixed point that the system approaches eventually
in time. Without loss of generality let us call Q the species for
which the concentration is zero at this attracting fixed point (clearly ![]() for the concentration to decrease in time from a positive value to zero).
Then, as before the solutions of (10)-(11)
are of the form (18)-(19),
but now
for the concentration to decrease in time from a positive value to zero).
Then, as before the solutions of (10)-(11)
are of the form (18)-(19),
but now ![]() . This implies that
. This implies that ![]() , at the fixed point
, at the fixed point ![]() ,
, ![]() , while
, while ![]() at the fixed point, unless
at the fixed point, unless ![]() , in which case it is
, in which case it is ![]() . Therefore, instead of Eqs. (23)-(24),
we get in this case:
. Therefore, instead of Eqs. (23)-(24),
we get in this case:
if ![]() , and
, and
if ![]() . The
. The ![]() case can be handled as before, yielding the reduced equations:
case can be handled as before, yielding the reduced equations:
On the other hand, in the ![]() case, if
case, if ![]() , Eq. (40) implies that
, Eq. (40) implies that ![]() varies linearly with T and this breaks down the asymptotic expansion
we are assuming. Therefore, we can separate the two timescales for the
k'=0,
varies linearly with T and this breaks down the asymptotic expansion
we are assuming. Therefore, we can separate the two timescales for the
k'=0, ![]() case as before only if
case as before only if ![]() , getting the reduced equations:
, getting the reduced equations:
In certain cases, a similar (but more complicated) calculation can be done if there is more than one fast reaction. Depending on the number of fast reactions and on the number of species involved, as many variables as fast reactions can be eliminated by this procedure. However, this is not always possible to accomplish and the situation has to be analyzed on a one by one basis.